Get full access with a free account
Benefits of the Coloplast® Professional Educational platform
![]() Get full access to all educational content, events and resources
Get full access to all educational content, events and resources
![]() Track your progress
Track your progress
![]() Share content with your colleagues
Share content with your colleagues
![]() Share supporting material with your patient
Share supporting material with your patient
Introducing Micro-hole Zone Technology
Micro-hole Zone Technology ensures** complete bladder emptying* in one free flow. By reducing mucosal suction, it eliminates the need for repositioning and reduces the risk of residual urine and microtrauma.1,2
Learn how Micro-hole Zone Technology works
Intermittent catheters with Micro-hole Zone Technology have 80+ micro-holes, designed to reduce the risk of UTIs†. Key benefits of Micro-hole Zone Technology are1:
- One free flow – The urine flow only stops when the bladder is completely emptied*
- No repositioning – There is no need to reposition the catheter as mucosal suction is reduced
- Less risk of UTIs – Designed to reduce the risk of UTIs† by minimizing residual urine and bladder microtrauma‡
*The Micro-hole Zone Technology catheter has close to no flow stops for complete bladder emptying, defined as <10 mL, Landauro, et. al, (2023), (N=42), when used in accordance with product 'Instructions for use'. Individual results may vary.
**When used in accordance to product 'Instructions for Use'
†UTI risk factors defined by Kennelly M et al. (2019), 10.1155/2019/2757862.3 Study supported by Coloplast
‡Tested in a pre-clinical setting (ex vivo). May not be indicative of clinical performance.
Drains the urine in one free flow
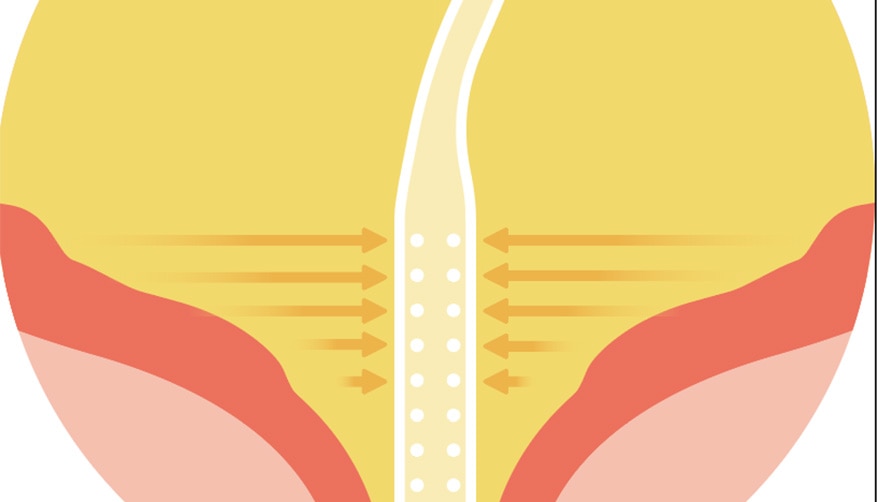
Designed to drain urine at the bottom of the bladder with 80+ micro-holes. 1
Two eyelets for urine draining.
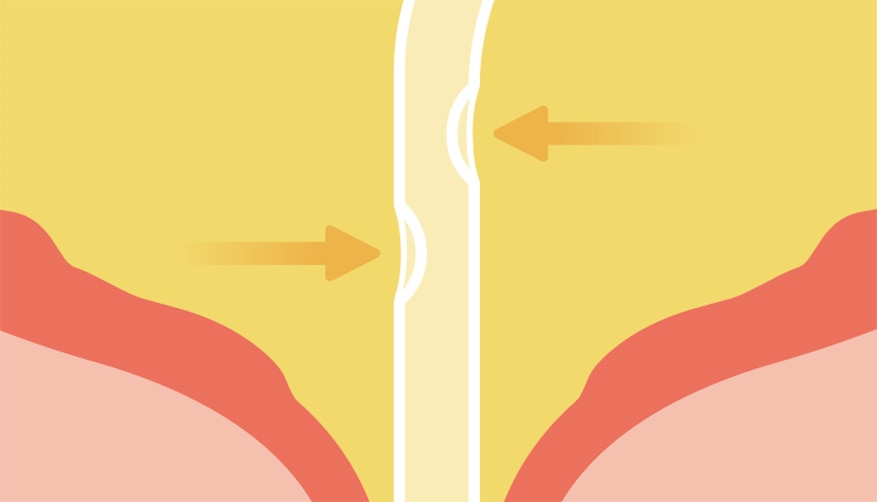
No need for repositioning

Reduces mucosal suction of the bladder wall. 1
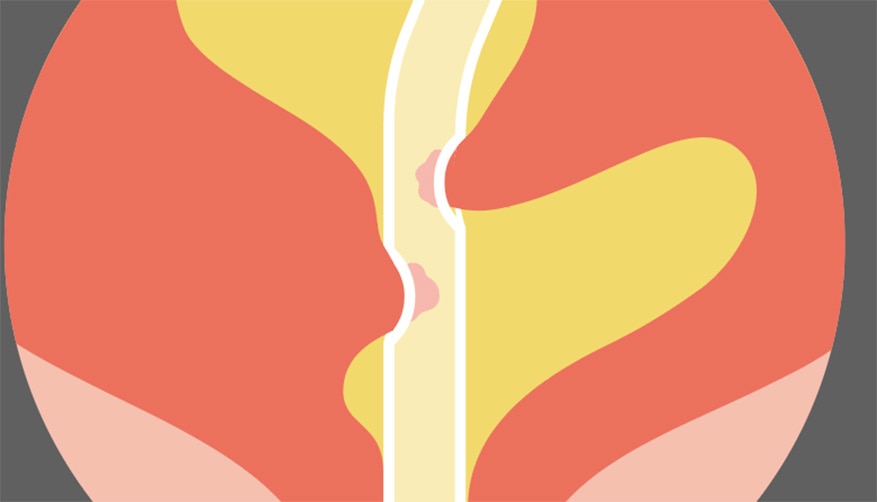
May lead to mucosal suction, causing the urine flow to stop. These flow stops may give a false indication of an emptied bladder. 2,4
Less risk of UTIs†
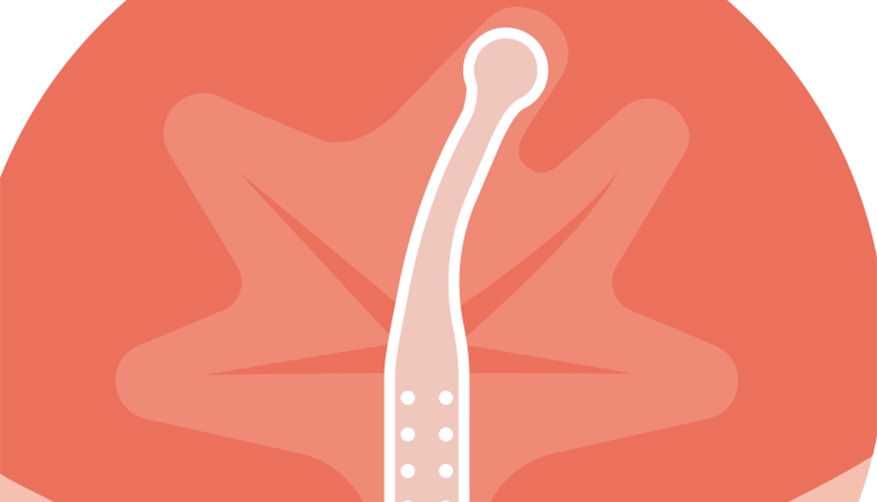
Is designed to reduce the risk of UTIs. 1,3
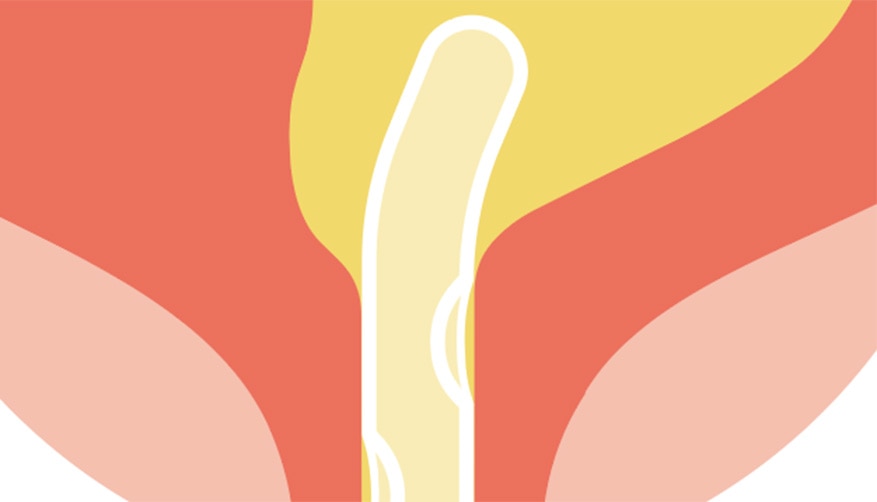
If residual urine is left behind in the bladder, bacteria may thrive and potentially lead to the development of UTIs. 5,6
What evidence is there about Micro-hole Zone Technology?
Click to learn more about the pre-clinical activities presented at global conferences.
Pre-clinical posters:
- Landauro MH, Jacobsen L, Tentor F, et al. New Intermittent Urinary Micro-Hole Zone Catheter Shows Enhanced Performance in Emptying the Bladder: A Randomised, Controlled Crossover Study. J. Clin. Med. 2023, 12, 5266.
- Tentor F, Schrøder BG, Nielsen S et al., Development of an ex-vivo porcine lower urinary tract model to evaluate the performance of urinary catheters. Scientific Reports. 2022; Oct 24;12(1):17818
- Kennelly M, Thiruchelvam N, Averbeck MA et al., Adult neurogenic lower urinary tract dysfunction and intermittent catheterisation in a community setting: Risk factors model for urinary tract infections. Advances in Urology. 2019; Apr 2;1–13. Study supported by Coloplast.
- Willumsen A, Rezaa T, Schertigera L, and Nielsen LF. Reduction in Eyelet Size in Intermittent Urinary Catheters Results in Less Urothelial Microtrauma in the Bladder. UKCS Annual Scientific Meeting; Sheffield, United Kingdom 2023.
- Vasudeva P and Madersbacher H, Factors implicated in pathogenesis of urinary tract infections in neurogenic bladders: some revered, few forgotten, others ignored. Neurology and Urodynamics. 2014 Jan;33(1):95-100
- Barber AE, Norton JP, Spivak AM et al., Urinary tract infections: current and emerging management strategies. Clinical Infectious Diseases. 2013; Sep;57(5):719-2
Prior to use, refer to product labeling for complete product instructions for use, contraindications, warnings and precautions.
Luja Coudé is indicated for use by patients with urine retention and patients with a post void residual volume (PVR) due to neurogenic and non-neurogenic voiding dysfunction. The catheter is inserted into the urethra to reach the bladder allowing urine to drain. The product is for adult male patients only. Apply with caution if the patient produces urine with many particles clearly distinguishable by the naked eye, as it may lead to transient urine retention. See the device manual for detailed information regarding the implant procedure, contraindications, warnings, precautions, and potential complications/adverse events. For further information, call Coloplast Corp. at 1-866-226-6362 and/or consult the company website at www.coloplast.us. Rx Only.
