Get full access with a free account
Benefits of the Coloplast® Professional Educational platform
![]() Get full access to all educational content, events and resources
Get full access to all educational content, events and resources
![]() Track your progress
Track your progress
![]() Share content with your colleagues
Share content with your colleagues
![]() Share supporting material with your patient
Share supporting material with your patient
Extensively studied. Consistent results. Gold standard procedure.
This compendium summarizes the clinical evidence for efficacy and safety of Restorelle Ultra Lightweight Mesh, which is indicated for use as a bridging material for sacrocolposuspension/sacrocolpopexy (transabdominal placement via laparotomy, laparoscopic, or robotic approach) where surgical treatment for vaginal vault prolapse is warranted.
Restorelle Y incorporates Smartmesh technology in customized shapes specifically for sacrocolpopexy procedures. It is the first mesh designed by a surgeon, specifically with a woman’s anatomy and tissue healing requirements in mind.

Ultra lightweight, plus long-term success rates
Restorelle redefined surgical outcomes and continues to restore patient anatomy and renew quality of life.

Salamon, International Urogynecology Journal 2013
Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy
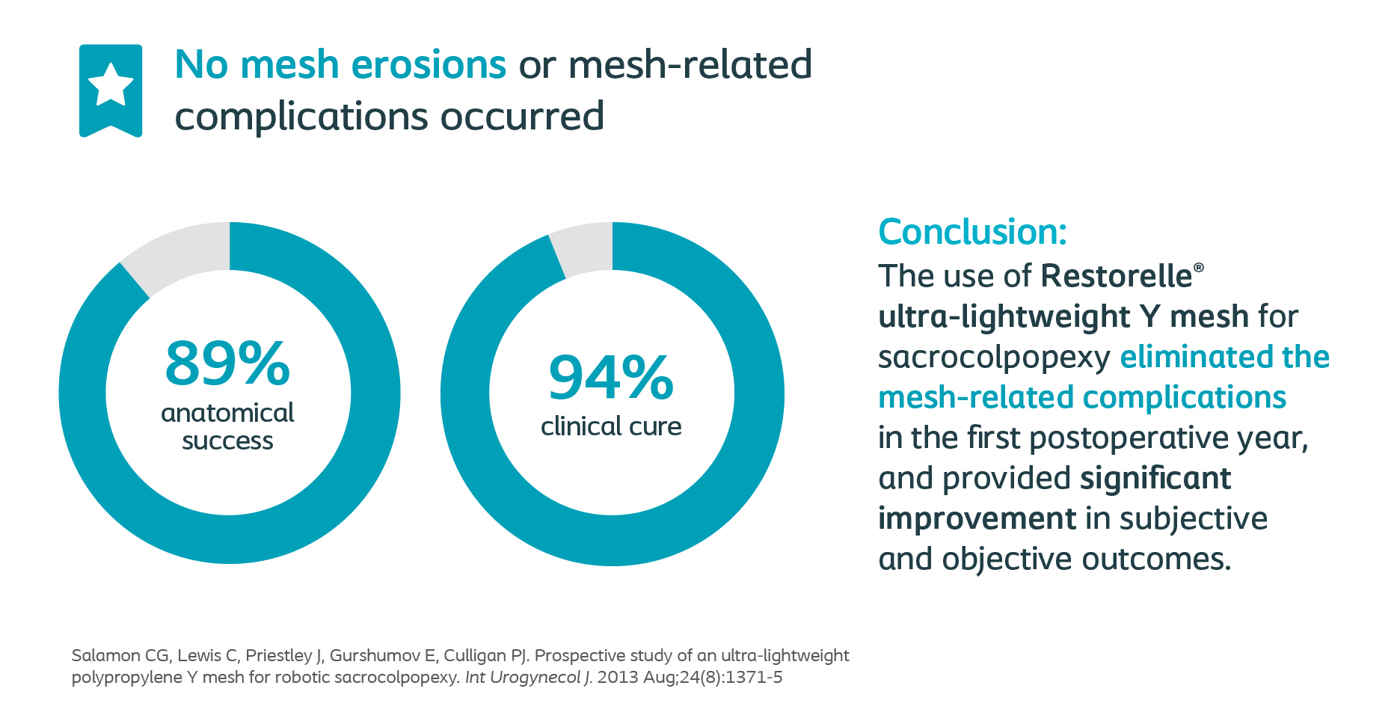

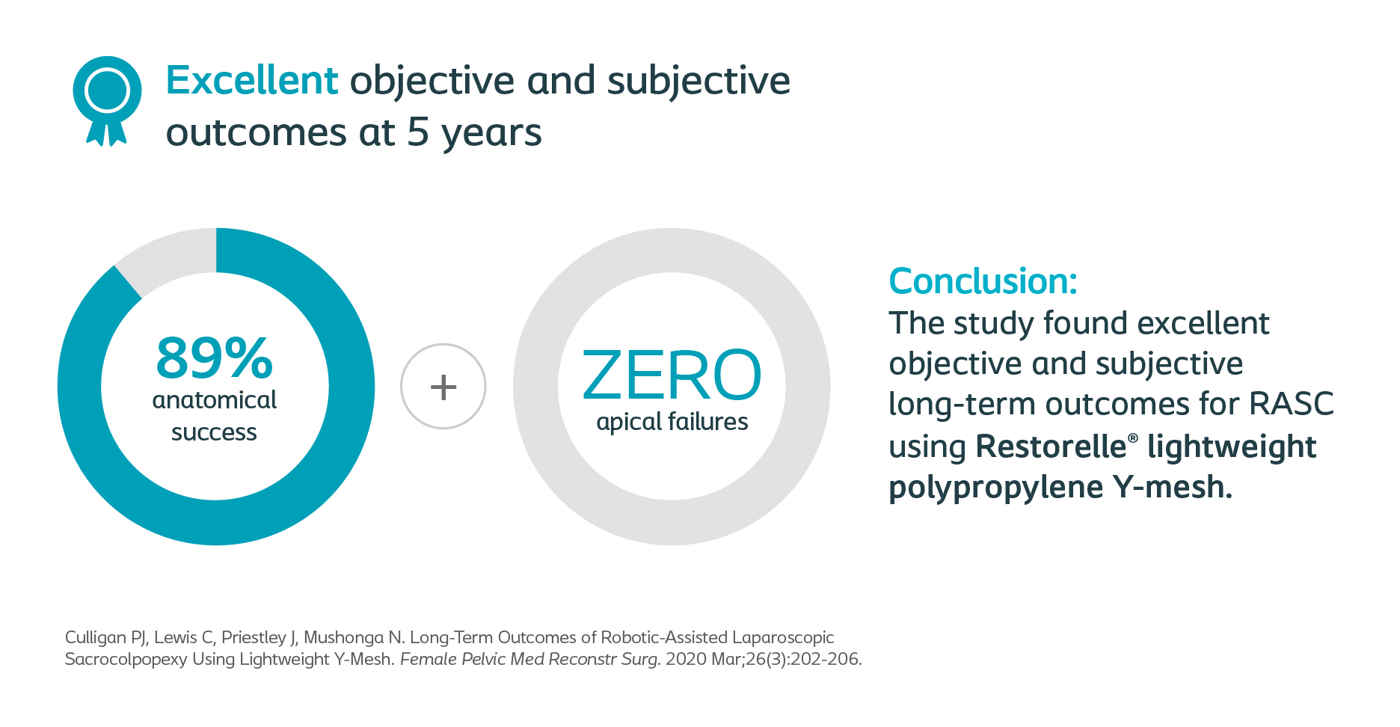
Culligan, Female Pelvic Medicine & Reconstructive Surgery 2020
Long-Term Outcomes of Robotic-Assisted Laparoscopic Sacrocolpopexy Using Lightweight Y-Mesh

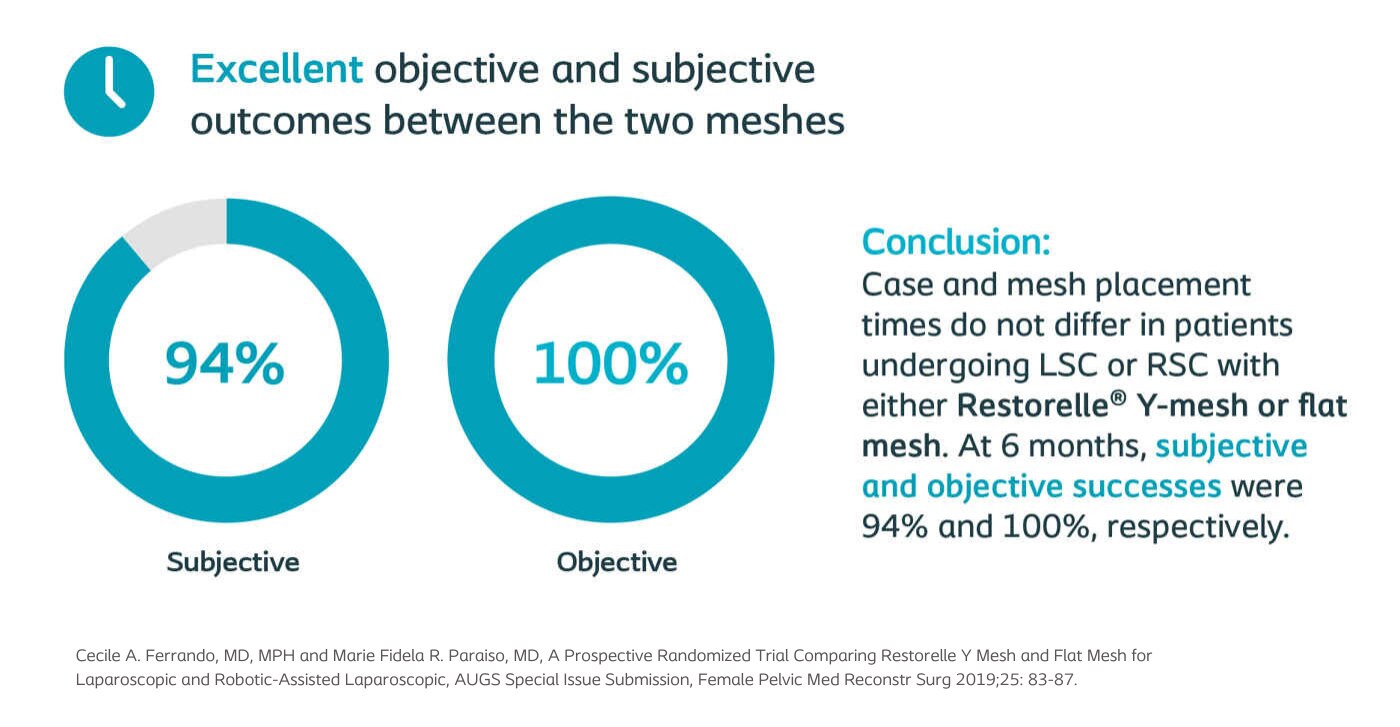
Ferrando, AUGS Special Issue Submission 2019
Prospective Randomized Trial Comparing Restorelle Y Mesh and Flat Mesh for Laparoscopic and Robotic-Assisted Ssacrocolpopexy
More published clinical studies
Assessing pelvic organ prolapse recurrence after minimally invasive sacrocolpopexy: does mesh weight matter?
Lead author: Giugale
Publication: International Urogynecology Journal; 2021
Coloplast sponsored? No
View study
Does Mesh Weight Affect Time to Failure After Robotic-Assisted Laparoscopic Sacrocolpopexy?
Lead author: Askew
Publication: Female Pelvic Medicine & Reconstructive Surgery; 2020
Coloplast sponsored? No
View study
Laparoscopic sacrocolpopexy (LSCP) using an ultra-lightweight polypropylene mesh
Lead author: Dwyer
Publication: European Journal of Obstetrics & Gynecology and Reproductive Biology; 2019
Coloplast sponsored? Yes
View study
Laparoscopic sacrocolpopexy posthysterectomy: intraoperative feasibility and safety in obese women compared with women of normal weight
Lead author: Mahoney
Publication: International Urogynecology Journal; 2019
Coloplast sponsored? Yes
View study
Polypropylene Mesh Predicts Mesh/Suture Exposure After Sacrocolpopexy Independent of Known Risk Factors: A Retrospective Case-Control Study
Lead author: Durst
Publication: International Urogynecology Journal; 2017
Coloplast sponsored? No
View study
Efficacy and Pregnancy Outcomes of Laparoscopic Single Sheet Mesh Sacrohysteropexy
Lead author: Pandeva
Publication: Neurourology and Urodynamics; 2017
Coloplast sponsored? No
View study
Titanium Surgical Tacks: Are They Safe? Do They Work?
Lead author: Shatkin-Margolis
Publication: Female Pelvic Medicine & Reconstructive Surgery; 2017
Coloplast sponsored? No
View study
Analysis of changes in sexual function in women undergoing pelvic organ prolapse repair with abdominal or vaginal approaches
Lead author: Gupta
Publication: International Urogynecology Journal; 2016
Coloplast sponsored? No
View study
Outcomes in 450 Women After Minimally Invasive Abdominal Sacrocolpopexy for Pelvic Organ Prolapse
Lead author: Mueller
Publication: Female Pelvic Medicine & Reconstructive Surgery; 2016
Coloplast sponsored? No
View study
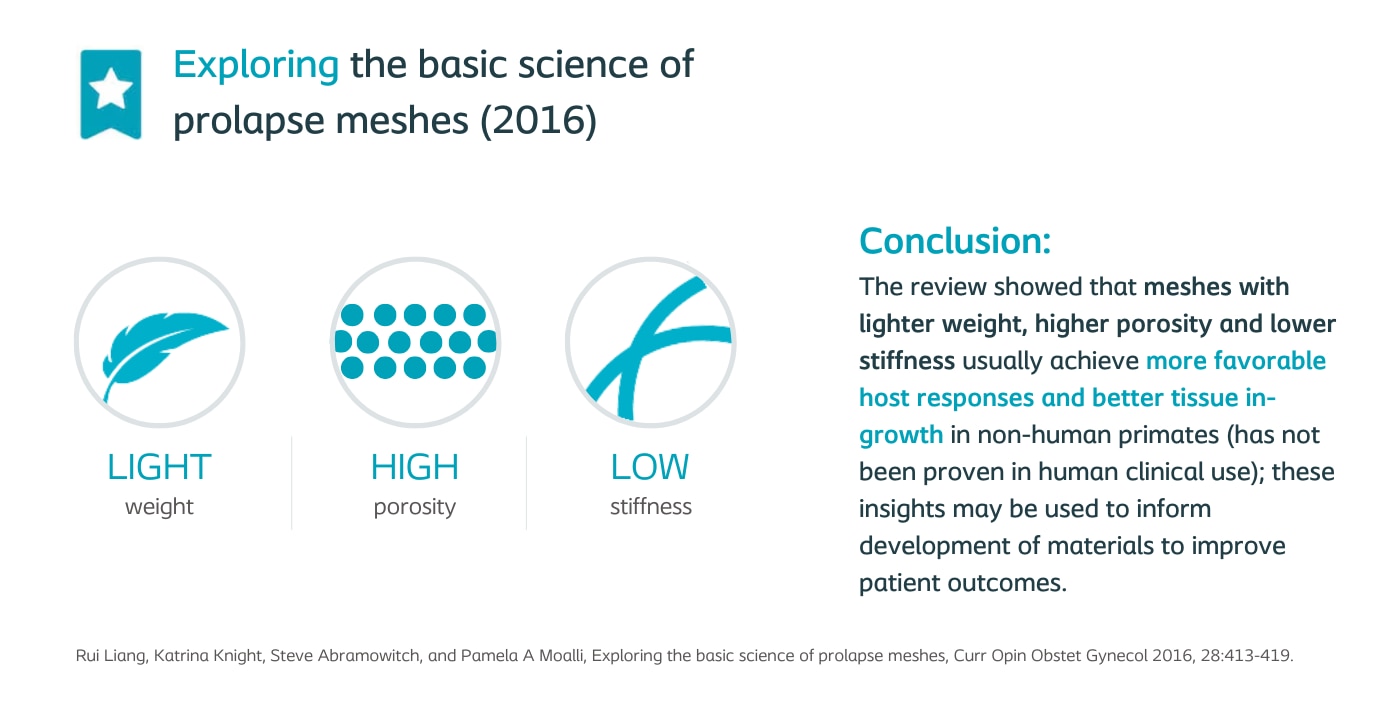
Exploring the basic science of prolapse meshes
Lead author: Liang
Publication: Current Opinion in Obstetrics and Gynecology; 2016
Coloplast sponsored? No
View study
Transvaginal Versus Transabdominal Placement of Synthetic Mesh at Time of Sacrocolpopexy
Lead author: Nosti
Publication: Female Pelvic Medicine & Reconstructive Surgery; 2015
Coloplast sponsored? No
View study
A randomized trial of vaginal mesh attachment techniques for minimally invasive sacrocolpopexy
Lead author: Tan-Kim
Publication: International Urogynecology Journal; 2015
Coloplast sponsored? No
View study
Safety and One Year Outcomes Following Vaginally Assisted Laparoscopic Uterine Sacropexy (VALUES) for Advanced Uterine Prolapse
Lead author: Fayyad
Publication: Neurourology and Urodynamics; 2013
Coloplast sponsored? No
View study
Short-Term Outcomes of Robotic Versus Conventional Laparoscopic Sacral Colpopexy
Lead author: Antosh
Publication: Female Pelvic Medicine & Reconstructive Surgery; 2012
Coloplast sponsored? No
View study
Optimizing Efficiency With Robot-Assisted Laparoscopic Sacrocolpopexy
Lead author: Salamon
Publication: The Female Patient; 2010
Coloplast sponsored? No
View study
Other publications
Restorelle M, L and XL, Restorelle Y, and Restorelle Y Contour Polypropylene Mesh Brief Statement
Indications
Restorelle M, L and XL, Restorelle Y, and Restorelle Y Contour are indicated for use as a bridging material for sacrocolposuspension/sacrocolpopexy (transabdominal placement via laparotomy, laparoscopic, or robotic approach) where surgical treatment for vaginal vault prolapse is warranted.
Contraindications
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the contraindications associated with the use of this product. The Restorelle M, L and XL, Restorelle Y, and Restorelle Y Contour are contraindicated for use in patients with the following conditions:
- Pregnancy or desire for future pregnancy
- Potential for further growth (e.g., adolescents)
- Pre-existing local or systemic infection. Treat the infection with the appropriate antiseptics and/or antibiotics to eliminate the infection before placing the Restorelle M, L, or XL, Restorelle Y, or Restorelle Y Contour mesh
- Taking anticoagulant therapy
- Any condition, including known or suspected pelvic pathology, which could compromise implant or implant placement
- Sensitivity/allergy to polypropylene
Warnings & Precautions
It is the responsibility of the physician to advise the prospective patients or their representatives, prior to surgery, of the warnings and precautions associated with the use of this product and the associated surgical risks.
The effectiveness of Restorelle Y Contour has not been validated by a prospective, randomized clinical trial.
Warnings
Restorelle M, L and XL, Restorelle Y, and Restorelle Y Contour mesh should only be used by physicians familiar with the surgical procedures and techniques involving non-absorbable mesh and who have adequate education and experience in the treatment of pelvic organ prolapse.
A thorough assessment of each patient should be made to determine the suitability of a synthetic mesh procedure.
The patient should be counseled that alternative prolapse treatments may be appropriate, and the reason for choosing a surgical mesh procedure should be explained.
Obtain patient consent prior to surgery and ensure that the patient has an understanding of the postoperative risks and potential complications of transabdominal mesh surgery.
Patient counseling should include a discussion that the mesh to be implanted is a permanent implant and that some complications associated with the implanted mesh may require additional surgery; repeat surgery may not resolve these complications. Serious adverse tissue responses or infection may require removal of mesh, and complete removal of the mesh may not always be possible. Individuals may have varying degrees of collagen laydown that may result in scarring.
Patient Warnings
As with all surgical procedures, patients with certain underlying conditions may be more susceptible to postoperative bleeding, impaired blood supply, compromised/delayed healing, or other complications and adverse events.
The risks and benefits of using Restorelle M, L and XL, Restorelle Y, and Restorelle Y Contour should be considered in patients with: • Age-related underlying conditions • Autoimmune disease • Coagulation disorder • Connective tissue disorder • Debilitated or immunocompromised state • Diabetes • Pelvic radiation therapy or chemotherapy • Physical characteristics (e.g., body mass index) • Smoking-related underlying conditions • Urinary tract anomalies.
Any future pregnancy could negate the benefits of this surgical procedure. Patients should report any bleeding, pain, abnormal vaginal discharge or sign of infection that occur at any time.
Procedure Warnings
Avoid placing excessive tension on the Restorelle M, L and XL, Restorelle Y, and Restorelle Y Contour mesh implant during placement and adjustment to maintain mesh integrity.
There should be an appropriate margin of mesh extending beyond the fixation points.
Inadequate fixation of the mesh material to the pelvic tissue may lead to failure of the repair and recurrence of the prolapse.
Procedure Precautions
Use caution to avoid neurovascular injury. Observe patient for any signs of abnormal bleeding or clinical signs of nerve damage.
Potential Complications
Adverse events are known to occur with transabdominal synthetic mesh procedures and implants. Adverse events following mesh implantation may be de novo, persistent, worsening, transient, or permanent.
Adverse events may include but are not limited to: • Abscess (acute or delayed) • Adhesion/scar formation • Allergy, hypersensitivity or other immune reaction • Bleeding, hemorrhage or hematoma • Bowel Related (Bowel obstruction, Constipation and/or defecatory dysfunction, Fecal incontinence and/or anal sphincter incompetence, Ileus) • Dehiscence • Delayed wound healing • Extrusion, erosion or exposure of mesh into the vagina or other structures or organs • Fistula formation • Infection • Inflammation (acute or chronic) • Local irritation • Mesh migration • Necrosis • Pain Related (De novo and/or worsening dyspareunia, Neuromuscular symptoms (acute or chronic), Pain (acute or chronic), Partner pain and/or discomfort during intercourse) • Perforation or injury of soft tissue (e.g., ligaments, muscles, nerves, vessels), structures, or organs (e.g., bowel, rectum, bladder, urethra, ureters, vagina) • Seroma • Suture erosion • Urinary Related (Bladder storage dysfunction (e.g., increased daytime frequency, urgency, nocturia, overactive bladder, urinary incontinence), Ureteral obstruction, Urinary tract infection, Voiding symptoms (e.g., dysuria, urinary retention, incomplete emptying, straining, positional voiding, weak stream) • Vaginal Related (De novo or worsening prolapse in untreated compartment, Granulation tissue formation, Palpable mesh (patient and/or partner), Recurrent prolapse, Sexual dysfunction, Vaginal discharge (abnormal), Vaginal scarring, tightening, rigidity, shortening and/or contracture.
The occurrence of adverse events may require one or more revision surgeries, including removal of the mesh. Complete removal of the mesh may not always be possible, and additional surgeries may not always fully correct the complications. There may be unresolved pain with or without mesh explantation.
The information provided is not comprehensive with regard to product risks. For a comprehensive listing of indications, contraindications, warnings, precautions, and adverse events refer to the product’s Instructions for Use. Alternatively, you may contact a Coloplast representative at 1-800-258-3476 and/or visit the company website at www.coloplast.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
PM-30439 02/24
